Predicting Metal-Dialkyl Compounds
Predicting Metal-Dialkyl Compounds Prone to Generate Alkylidenes from Carbon-13 Chemical Shift
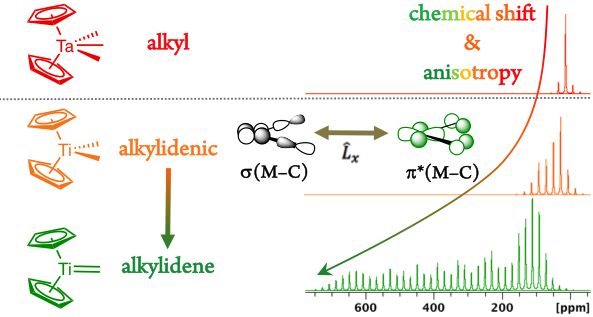
NMR chemical shift encodes information on molecular reactivity of organometallic intermediates. Here, we demonstrate that metal di-alkyl compounds that are prone to generate alkylidenes show distinctive deshielded chemical shifts. These chemical shift values originate from the presence of a low lying empty metal orbital, ideally located to promote α-H abstraction.
Access the paper published in external pageChemical Science.call_made