Electronic Structure–Reactivity Relationship
Electronic Structure–Reactivity Relationship on Ruthenium Step-Edge Sites from Carbonyl 13C Chemical Shift Analysis
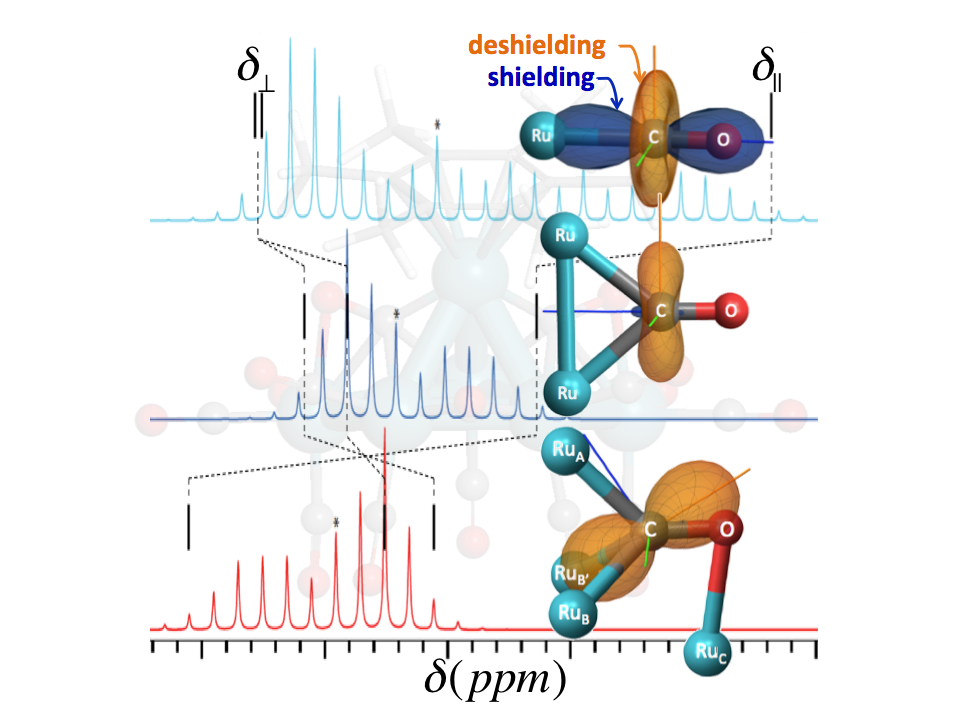
Ru nanoparticles are highly active catalysts for the Fischer–Tropsch and the Haber–Bosch processes. They show various types of surface sites upon CO adsorption according to NMR spectroscopy. Compared to terminal and bridging adsorption modes on terraces or edges, little is known about side-on CO species coordinated to B5 or B6 step-edges, the proposed active sites for CO and N2 cleavage. By using solid-state NMR and DFT calculations, we analyzed 13C chemical shift tensors (CSTs) of carbonyl ligands on the molecular cluster model for Ru nanoparticles, Ru6(η2-μ4-CO)2(CO)13(η6-C6Me6) and showed that the CST principal component parallel to the C–O bond is extremely deshielded in the side-on species due to the population of the C–O π* antibonding orbital, which weakens the bond prior to dissociation. The carbonyl CST is thus an indicator of the reactivity of both Ru clusters and Ru nanoparticles step-edge sites toward C–O bond cleavage.
Access the paper published in external pageThe Journal of Physical Chemistry Letterscall_made.